R&D
Introduction
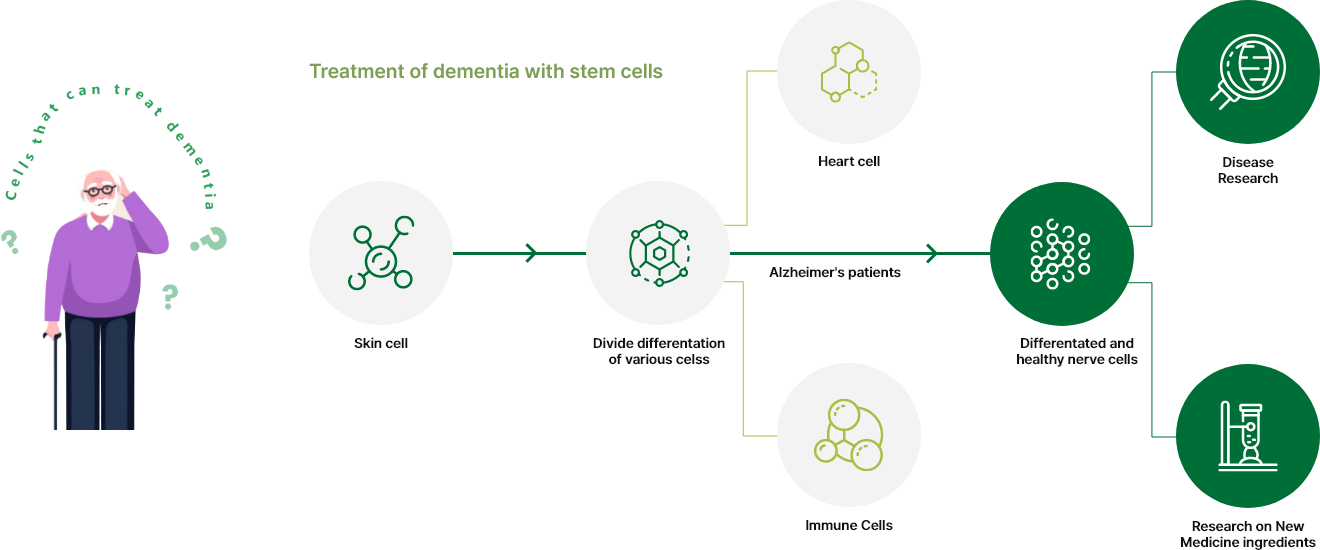
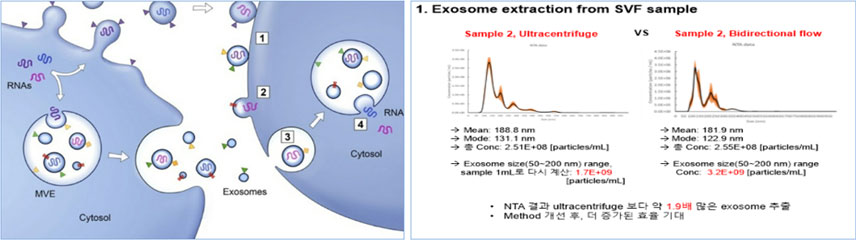
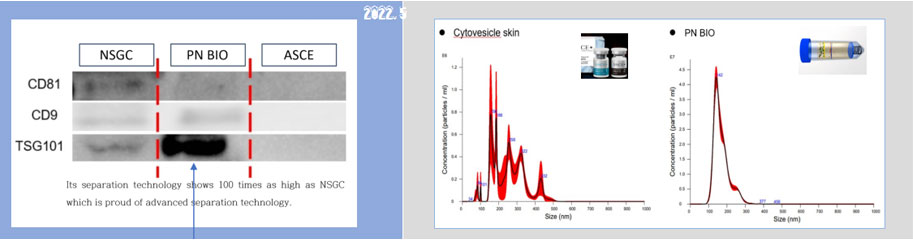
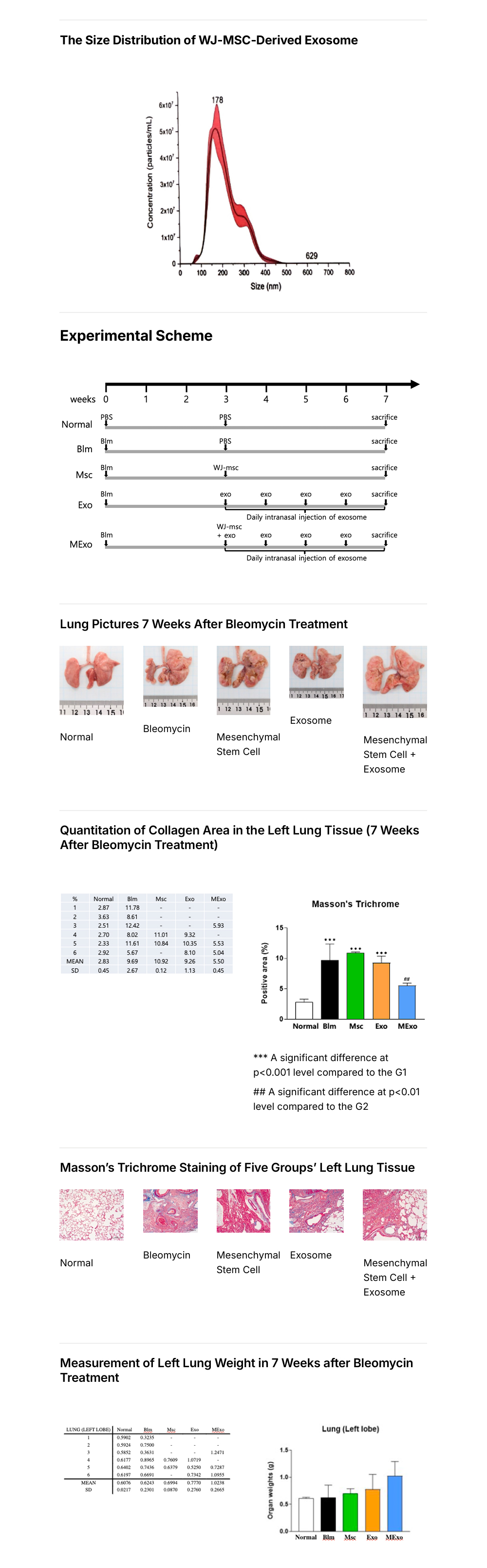
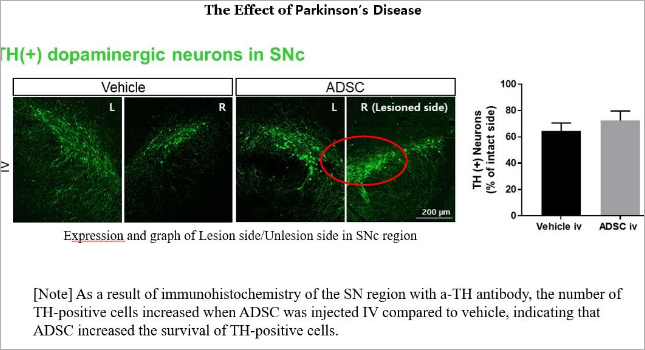
The PANACELL BIOTECH Research Center has the separation capabilities of
high -purity ADSC, NK Cell, Exosome, and Brown FAT,
which runs through the entire process of cell therapy, as well as the technologies
that track various differentiation of stem cells.
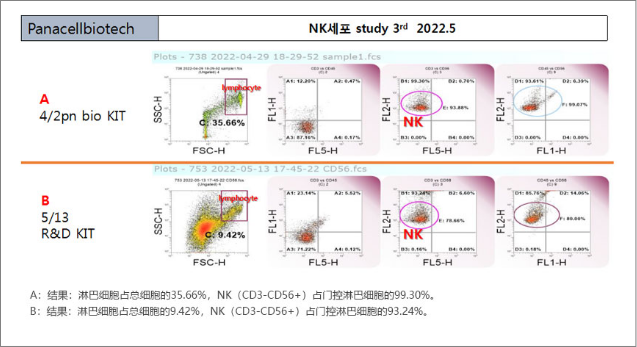
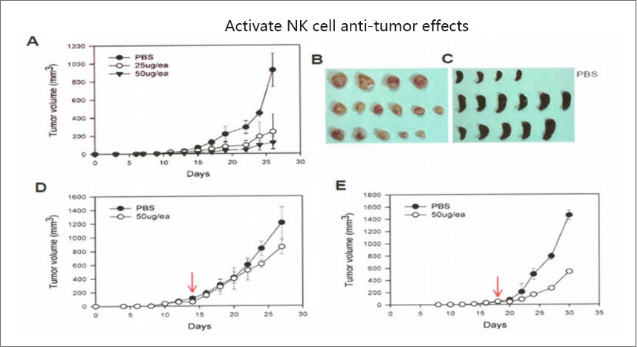
After MNC extraction NK cells of Negative Selection are used for a lot of culture.
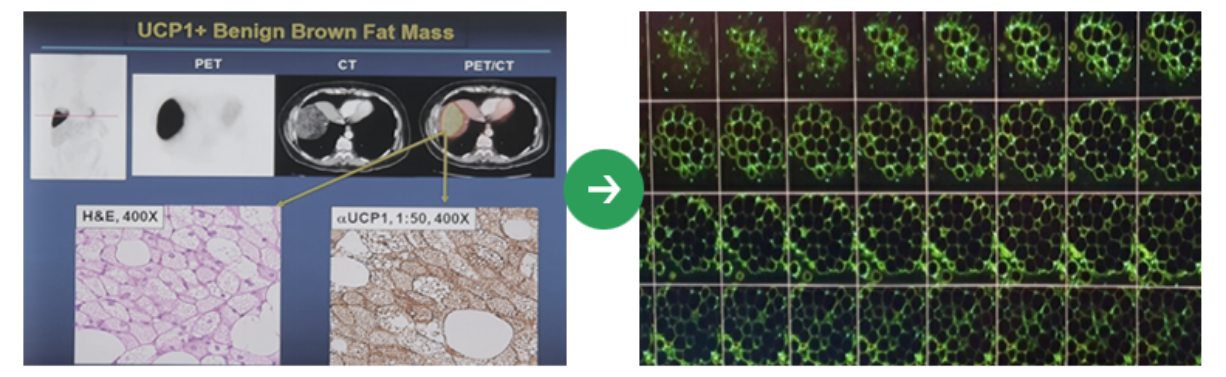
Ensure that world -class fat stem cells differentiate into cross -differentiated
technology for brown fat stem cells.








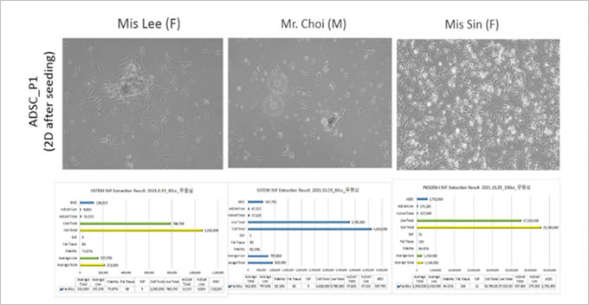
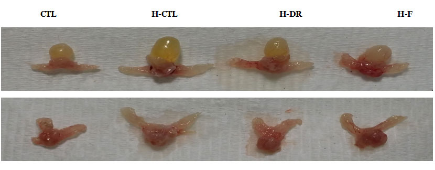


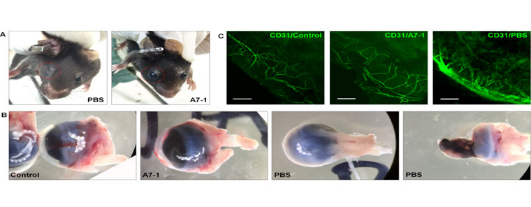



















 +82-2-593-3210
+82-2-593-3210 info@panacellbio.com
info@panacellbio.com 3F, Inspire Ent. Resort, 127 Jung-gu, Incheon, Republic of Korea
3F, Inspire Ent. Resort, 127 Jung-gu, Incheon, Republic of Korea